Background: Warfarin remains the anticoagulant of choice for patients with antiphospholipid syndrome (APLS). However, conflicting data exists regarding the role of direct oral anticoagulants (DOACs) as reasonable alternatives to warfarin, which has a less desirable side effect profile. In clinical practice, some patients start taking DOACs or aspirin before an official APLS diagnosis is confirmed and these patients may choose to continue a DOAC after an informed discussion with their hematologist. To gain a better understanding of anticoagulant use in APLS at our center, we conducted a retrospective study to assess the clinical characteristics of patients diagnosed with APLS.
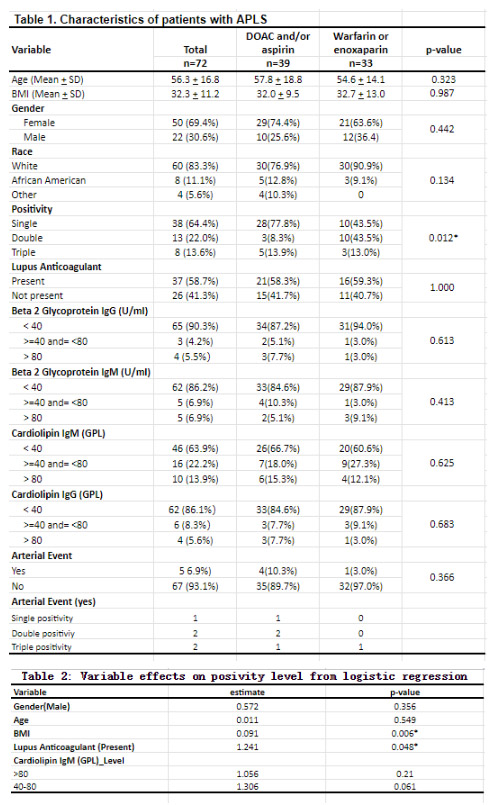
Methods: All patients with an ICD-10 code indicating APLS from January of 2020 to April of 2023 were included. Patient characteristics, relevant laboratory testing (lupus anticoagulant, beta-2 glycoprotein IgG/IgM, and anti-cardiolipin IgG/IgM), and treatment history were extracted from the electronic medical record. Patients were deemed to have single positivity if any one of the following were present: detectable lupus anticoagulant, elevated (defined as >40 GPL) beta-2 glycoprotein (B2GP) IgG or IgM, or elevated cardiolipin IgG or IgM both on initial and confirmatory testing (performed approximately 12 weeks later). Similarly, double positivity was defined as the presence of two of these biomarkers and triple positive was defined as the presence of three of these biomarkers. Initial laboratory testing results are included in Table 1. Statistical analysis was conducted using the SAS software (SAS Enterprise Guide 7.1) and the R Core Team (R 4.2.2). Continuous variables such as BMI and age were evaluated using the Wilcoxon rank-sum test, while categorical variables were assessed using Fisher's Exact Test and Chi-squared test.
Results: Seventy-two patients were included in our analysis. Mean age and BMI were 56.3 ± 16.8 years and 32.3 ± 11.2, respectively. The majority of patients were female (69.4%) and white (83.8%). Approximately half of all patients had single positivity (64.4%) and more than half had detectable lupus anticoagulant (58.7%). Cardiolipin IgM was the most frequently (36.1%) elevated biomarker. Patient characteristics between the DOAC/aspirin and warfarin/enoxaparin cohorts were not significantly different; however, significantly more patients with single positivity received DOAC/Aspirin rather than warfarin/enoxaparin (77.8% vs.43.5%, p = 0.012). There was no statistically significant difference between the frequency of arterial complications in patients who took DOAC/Aspirin (4 events in 39 patients) versus those who took warfarin/enoxaparin (1 event in 33 patients), p = 0.366. The majority of arterial complications (4 of 5 events) occurred in patients with double and triple positivity. In addition, a logistical regression was performed regarding different characteristic effects on positivity level (single, double or triple laboratory positive), which found the presence of lupus anticoagulant had a significant association with higher likelihood of double/triple positivity (p = 0.048).
Conclusion: This study provides potential reassurance for clinicians regarding the use of DOACs during the investigative phase of APLS and supports the continuation of DOAC therapy in patients, particularly those with single positivity, after careful evaluation and shared decision-making with patients. However, caution should be kept when considering DOAC use in patients with lupus anticoagulant, double, or triple positivity, due to concerning arterial events. Further large multi-center randomized clinical trials are necessary in the future to clarify the role of DOACs in APLS.
Disclosures
No relevant conflicts of interest to declare.